University of Geneva researchers uncover two distinct origins of key brain cells
11 August 2025
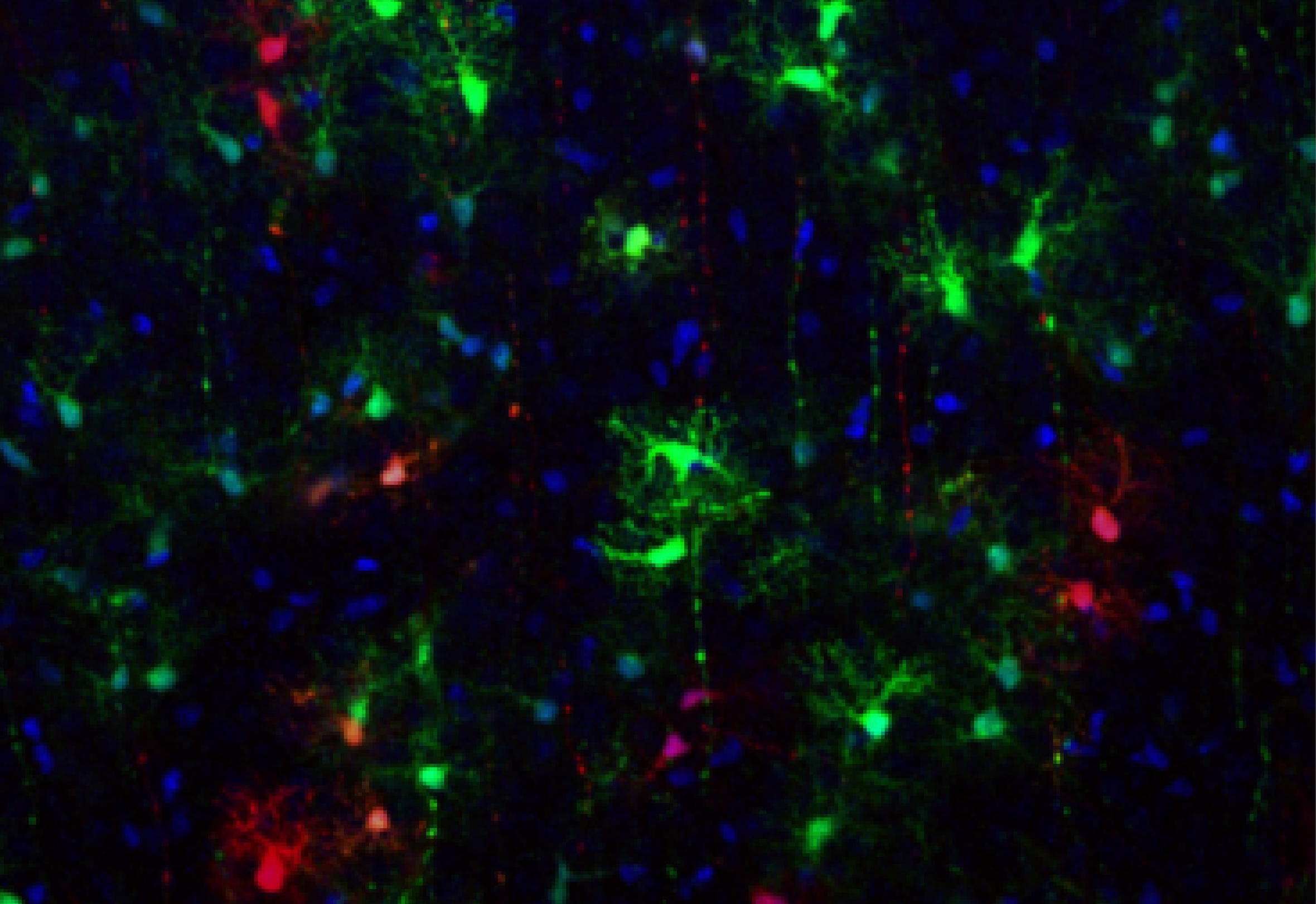 The brain’s cortical astrocytes are a varied, not uniform, cell population. | © R.Bocchi
The brain’s cortical astrocytes are a varied, not uniform, cell population. | © R.Bocchi
Researchers at the University of Geneva have uncovered that astrocytes in the brain’s cortex originate from two distinct types of progenitors, a finding that could reshape therapeutic strategies for neurological disorders.
Astrocytes, once thought to be a uniform group of passive support cells, are now recognized as key players in the central nervous system. They contribute to the blood–brain barrier, support neuronal metabolism, and regulate synaptic connectivity. Their involvement in a wide range of psychiatric and neurodegenerative conditions has prompted scientists to examine whether they form a homogeneous population or comprise multiple specialised subtypes.
A research team led by Riccardo Bocchi, Ambizione researcher at the Faculty of Medicine and its Synapsy Centre, has previously identified five distinct astrocyte subtypes in the mouse cortex, each located in specific cortical regions. Their new study, published in Nature Communications, addresses a fundamental question : how does this diversity arise during development ?
The team’s findings reveal that cortical astrocytes originate from two different progenitor lineages. The first, Emx1⁺ progenitors, were already known to produce neurons during embryonic development and then generate astrocytes closer to birth. The second lineage, marked by the transcription factor Olig2, produces a separate class of astrocytes with unique functions.
Functional differences between these lineages are significant. Olig2-derived astrocytes play a key role in forming new synapses, essential for building healthy neural circuits. In experiments where Olig2 was deactivated in mice, researchers observed not only a reduction in this astrocyte subtype but also a marked decrease in synapse numbers, underscoring their role in synaptogenesis.
New insights that could guide targeted therapies for brain disorders
The discovery carries important therapeutic implications. For instance, cell reprogramming strategies aiming to convert astrocytes into neurons, to replace those lost after events such as stroke, could be more effective if they focus on Emx1⁺-derived astrocytes, which share a developmental lineage with neurons. Conversely, targeting Olig2-derived astrocytes might help restore synaptic connections lost in certain psychiatric conditions.
By revealing the developmental origins and distinct functions of astrocyte subtypes, the University of Geneva team offers a new framework for classifying and manipulating these cells in the brain. As Bocchi notes, understanding astrocyte diversity is a critical step towards developing tailored interventions for neurological and psychiatric disorders.