Aspivix launches its breakthrough Carevix device in the United States
21 October 2025
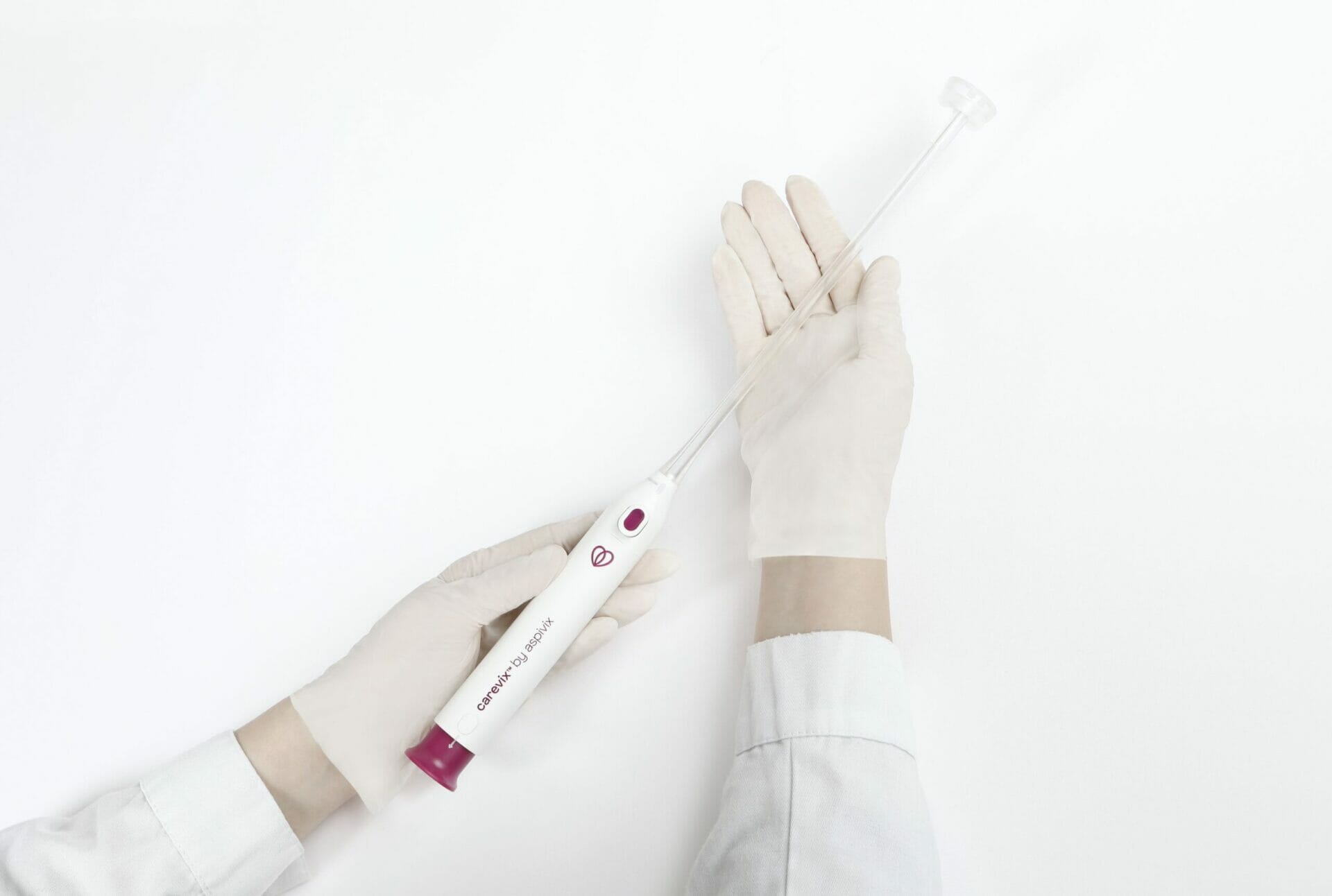 Aspivix’s Carevix device offers a gentler alternative to the century-old tenaculum, using suction instead of sharp forceps to reduce pain and bleeding during gynecological procedures. | © Aspivix
Aspivix’s Carevix device offers a gentler alternative to the century-old tenaculum, using suction instead of sharp forceps to reduce pain and bleeding during gynecological procedures. | © Aspivix
Vaud-based FemTech company Aspivix begins commercial rollout in Southern California and New York following strong clinical validation and growing U.S. demand.
Aspivix, the Lausanne-based FemTech innovator transforming gynecological care, has officially launched its Carevix device in the United States. The company’s suction-based cervical stabilizer, a modern alternative to the century-old tenaculum, is now available to physicians in Southern California and New York, marking a major milestone in its U.S. commercialization strategy.
Carevix uses gentle suction instead of sharp forceps to stabilize the cervix during intrauterine device (IUD) insertions and other gynecological procedures. Clinical data show the device reduces pain by over 70% and bleeding by more than 80% compared to traditional tools, providing a significant advance in women’s health comfort and safety.
The U.S. launch follows years of development and validation, including FDA clearance, CE marking, and recognition by TIME magazine as one of the 200 Best Inventions of 2024. A new report in BMJ Sexual & Reproductive Health further confirmed Carevix’s clinical value, highlighting high satisfaction rates among both patients and healthcare providers in Sweden.
“As we launch in Los Angeles, Orange County, and New York, we’re not just introducing a new device — we’re redefining the standard of gynecological care,” said Mathieu Horras, CEO and co-founder of Aspivix.
Advancing women’s healthcare innovation worldwide
Aspivix’s expansion builds on successful pilot programs with Indiana University Health, Columbia University, and Tia Health. Over 1,200 procedures across nine countries provided real-world evidence supporting Carevix’s safety, efficacy, and ease of adoption.
In the U.S., healthcare professionals can now order Carevix directly through the new portal myaspivix.us, with training and on-site support available in both regions.
“We’re giving women and people with a uterus access to a gentler, more respectful experience, because gynecological pain can and should be reduced,” said Ikram Guerd, General Manager U.S. of Aspivix.