Compremium launches human study for non-invasive thyroid cancer diagnostic
15 July 2025
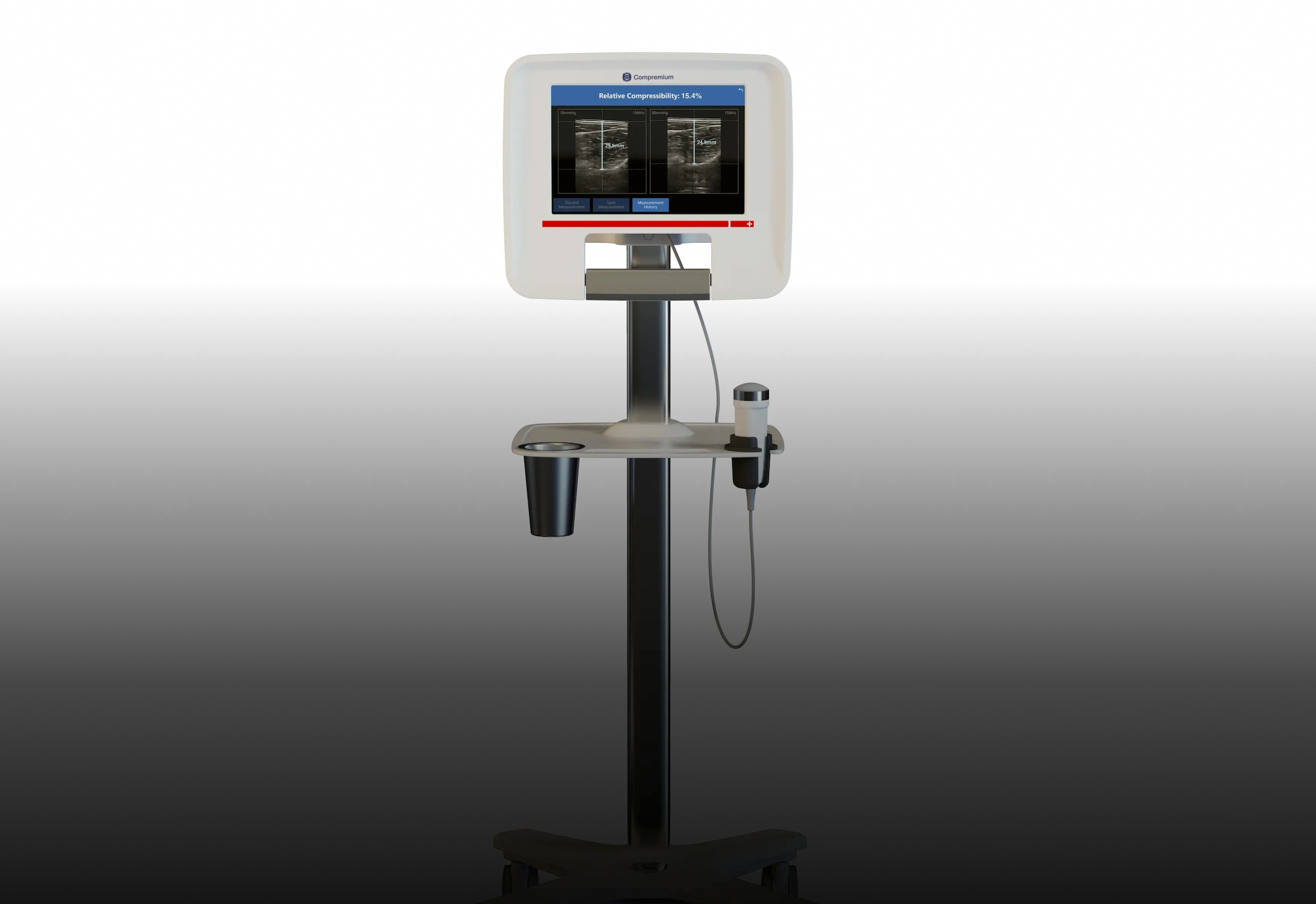 Compremium’s device measures tissue compressibility to distinguish between benign and malignant thyroid nodules. | © Compremium
Compremium’s device measures tissue compressibility to distinguish between benign and malignant thyroid nodules. | © Compremium
Bern-based medtech Compremium is testing its compressibility-based device in patients with thyroid nodules to improve diagnostic precision and reduce unnecessary surgeries.
Compremium has initiated its first-in-human clinical study to assess a novel, non-invasive diagnostic device for thyroid nodules. The study, conducted in collaboration with Bern University Hospital (Inselspital), aims to evaluate whether measuring tissue compressibility can improve clinical decision-making in thyroid cancer diagnostics.
The trial targets patients with Bethesda IV thyroid nodules, a group with an intermediate risk of malignancy, for whom current diagnostic methods often fail to provide clear guidance. Many of these patients undergo surgery as a precaution, though only 25% to 40% of nodules are ultimately found to be cancerous. By offering a more precise, non-invasive assessment, Compremium’s device could help reduce the number of unnecessary thyroidectomies.
Led by a multidisciplinary team including Prof. Marco Caversaccio, Dr. Urs Borner, and Dr. Samuel Tschopp, the 18-month study will enroll up to 30 adult patients scheduled for surgery. These patients will undergo additional compressibility testing using Compremium’s CPMX2 system, which enables real-time visualization of tissue stiffness.
Thyroid nodules affect up to two-thirds of adults, but fewer than 5% are malignant. Fine needle aspiration, the current diagnostic standard, is limited in its ability to classify indeterminate nodules. “This new technology might help us make more accurate and individualized clinical decisions,” said Dr. Borner.
Promise beyond thyroid care
Compremium’s proprietary platform integrates ultrasound imaging with pressure sensing to provide a real-time picture of tissue compressibility—malignant tissue generally being firmer than benign. Already FDA-cleared for other applications, the technology has been validated in over 40 clinical studies and shows potential across more than 30 medical indications.
“This study allows us to explore a critical application of our technology,” said Vincent Baumann, CEO of Compremium. “We believe this approach could significantly improve how thyroid nodules are evaluated and guide treatment decisions more effectively.”
If successful, the study could pave the way for broader use of Compremium’s device in oncology and other diagnostic domains. The trial is registered in the Human Research Switzerland database.