Novostia to launch first-in-human trials of its innovative artificial heart valve
8 May 2023
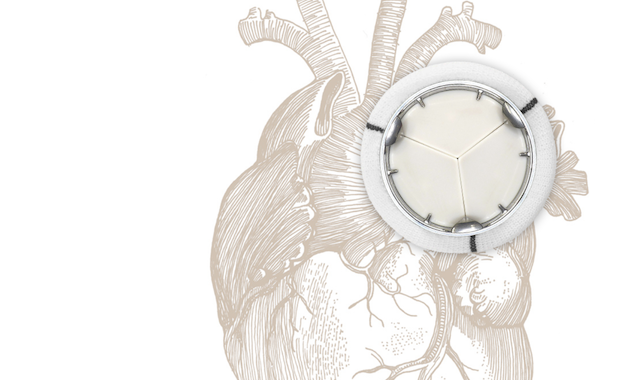
Novostia, a clinical-stage company focused on developing breakthrough artificial heart valves, recently announced the successful completion of its Series A financing round, raising EUR 2.5 million.
Novostia is a clinical-stage company focused on the development of innovative artificial heart valve technology. Their mission is to improve the quality of life for patients suffering from heart valve diseases by eliminating the drawbacks associated with existing valve replacement technologies.
Heart valve diseases affect over 100 million people worldwide, with hundreds of thousands of patients undergoing heart valve replacement procedures each year. Existing technologies present serious drawbacks, such as the need for lifelong anticoagulant medication or further replacements due to limited valve durability. Utilizing a unique patented design and a high-performance biocompatible polymer, Novostia’s artificial heart valve aims to function without the need for anticoagulation therapy and provide enhanced durability for patients of all ages.
Novostia was founded in April 2017 in Neuchâtel, a region renowned for its innovative technology and biomedical industries. The company was created at the Microcity incubator, which provided it with a supportive environment for the development of its groundbreaking artificial heart valve technology.
Located today on the premises of the Biopôle campus, Novostia will use the proceeds from the Series A round to initiate First-in-Human (FIH) trials for its innovative artificial heart valve. A Series B financing round is planned for this summer, following a re-evaluation of the company. The Series B funds will be used to complete the Pilot Study and launch the subsequent Pivotal Study, which will enroll around 60 patients for the former and 500 for the latter. These studies are expected to provide the necessary data for Novostia to obtain CE mark and Pre-Market Approval, allowing them to enter the market.
Revolutionizing heart valve replacement
Experimental, numerical, and animal testing have shown that the Novostia valve operates physiologically like a native human heart valve. It does not produce high-velocity backflow jets, does not elicit a hemostatic response, and is expected to function without the need for anticoagulation therapy. Unlike tissue valves, which have limited durability, especially in younger patients, the Novostia valve is structurally designed to last a lifetime for patients of any age, significantly reducing the risk of re-operation.
This innovative artificial heart valve technology has the potential to significantly improve patients’ quality of life, particularly for children and young adults, while also reducing healthcare system costs. The successful Series A funding round is an essential step toward bringing this long-awaited innovation to patients and physicians worldwide.