The import of foreign products into Switzerland: some standards to respect

Thanks to the various agreements that bind Switzerland to many countries, the import of foreign products is largely facilitated. However, there are a few standards that must be respected when importing foreign products into the country. These standards vary depending on the category of the product concerned.
For reasons of health and safety, environmental and consumer protection as well as to comply with international and national standards, certain products are subject to special requirements before they can be imported and marketed in Switzerland (for example drugs, cosmetics, or cleaning products).
It is the legislator who decides, on the basis of a product’s hazard potential, which conformity assessment procedures must be applied. These can be carried out by:
- The manufacturer itself (e.g. for machines)
- The competent public authority (e.g. for medicines)
- An independent third party (e.g. conformity assessment bodies)
These procedures are important because they allow the consumer’s protection and the guarantee of the products’ quality imported into Switzerland. However, the laws and regulations governing the import of these products vary depending on the exporting country and the type of product imported. Below are some particular cases to be aware of when importing products into Switzerland.
Agreements and principles that facilitate the import of foreign products into Switzerland
There are agreements between Switzerland and foreign countries that facilitate international trade, and, therefore, the import of foreign products into Switzerland. The main agreements are the Mutual Recognition Agreements (MRAs), recognized within the framework of the World Trade Organization (WTO). They aim, among other things, to remove technical trade barriers in the state-regulated sector. Thus, if the product regulations in two states are of a comparable standard, a conformity assessment conducted under the exporting country’s regulations is sufficient to allow the product to be distributed in the other country. Conversely, if the technical requirements differ between the two countries concerned, the producer must produce different series for the two destination markets.
The most important MRA for Switzerland in terms of economic policy is the one with the European Union (recognizable by the acronym CE which stands for Conformité Européenne). By largely adopting the EU’s rules in the area of product safety, Switzerland facilitates exports and imports between itself and the EU. However, there are also MRAs between Switzerland and countries outside the EU:
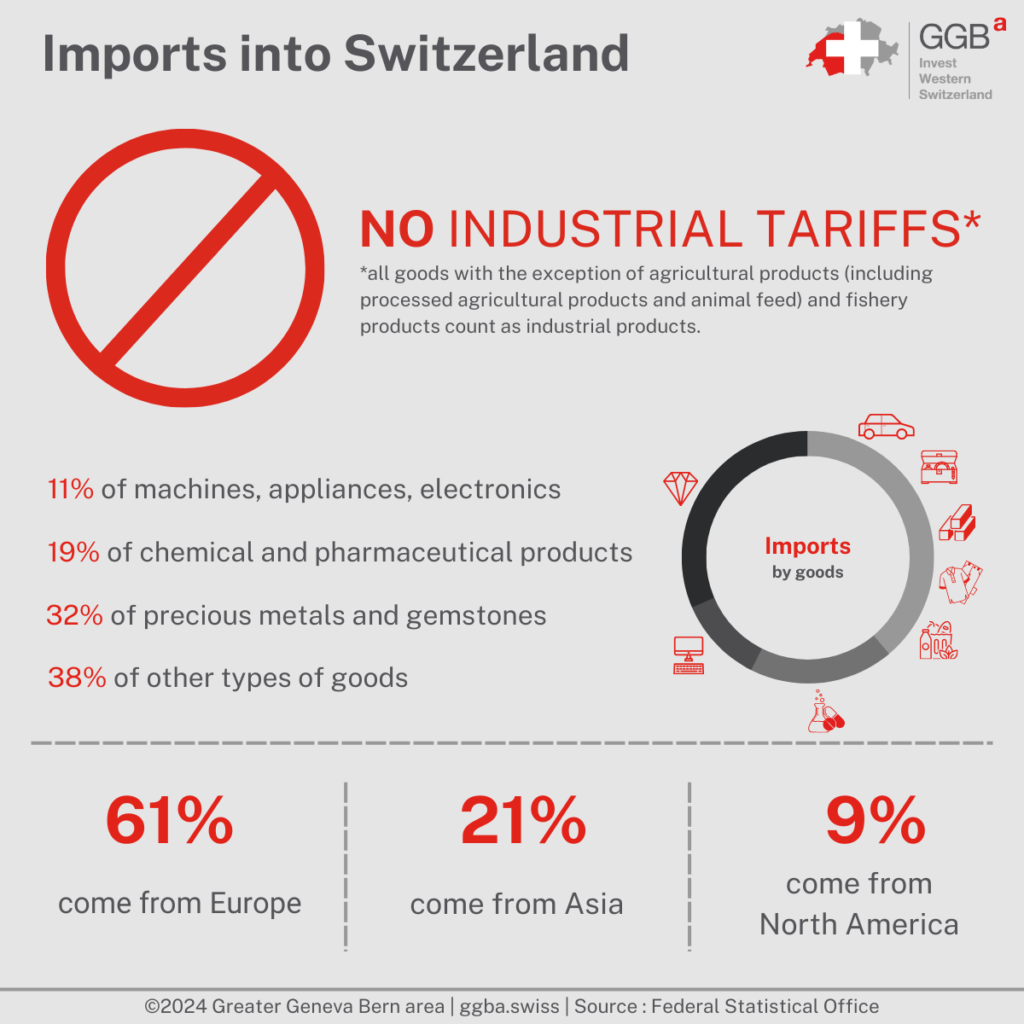
Starting from 1 January 2024, Switzerland sees a significant change in its trade policy with the abolition of industrial tariffs. This decision, announced by the Federal Council, marks a pivotal shift towards enhancing the competitiveness of Swiss businesses and reducing the cost of imports. The removal of these tariffs is expected to benefit both the industrial sector and consumers, as it lowers the cost of foreign products, including consumer goods such as cars, bicycles, personal care products, household appliances, and clothing. This move is a part of Switzerland’s ongoing efforts to reduce trade barriers and streamline import processes, complementing existing agreements such as the MRAs and the Cassis de Dijon Principle. The Cassis de Dijon Principle that allows products from the EU and the European Economic Area (EEA) to be imported into Switzerland without having to be repacked or re-labeled for the Swiss market. This is only the case if the products meet the regulations of the relevant EU or EEA country and were circulated there lawfully.
Finally, it is important to emphasize that Swiss product liability regulations largely correspond to those in force in the EU: the manufacturer is objectively liable for damage caused by defective goods. Therefore, it is essential to know the laws and ordinances governing the importation of foreign products into Switzerland, depending on the imported product’s category. You will find below the particularities of the categories of products mainly imported into Switzerland: it is nevertheless possible to find additional information concerning other types of products on the Swiss Confederation’s official website.
Foodstuffs
The Swiss Ordinance on the Identification and Advertising of Foodstuffs (ODAIOUs) contains strict regulations on the information that must be declared for foodstuffs:
- All ingredients must be named and listed on the packaging or labels of pre-packed foodstuffs in descending order of quantity.
- Foodstuffs which are not defined in a federal ordinance must be approved by the Federal Office of Public Health (FOPH).
- Foodstuffs, additives and processing agents which are genetically modified organisms (GMOs), contain such or have been obtained from such, and which are intended for offering to consumers are also subject to authorization by the FOPH. It is important to know that the existence of GMOs is tolerated if the share of an ingredient does not exceed 0.9%. Beyond this percentage, the product is subject to authorization.
- Nutritional and health claims must comply with the legal provisions in accordance with the ODAIOUs.
- No product marketed as foodstuffs or special foodstuffs may claim curative properties. Products with healing properties are medicines and are therefore subject to authorization by the Swiss Agency for Therapeutic Products Swissmedic.
It should be noted that there is a special regime decreed by the Swiss Parliament in the framework of the Cassis de Dijon Principle for foodstuffs. Indeed, foodstuffs from abroad that do not completely correspond to Switzerland’s technical specifications must be submitted to the FOPH for authorization.
Pharmaceutical products
The manufacturing and sales of drugs in Switzerland are always subject to mandatory licensing. To license a new pharmaceutical product, it is necessary to apply to Swissmedic. Normal evaluation of a license application for a human medicine with a new active ingredient costs CHF 80,000, and CHF 30,000 for medicinal products with an active agent recognized as innovative. As the requirements are largely similar to those of the EU, the simultaneous submission of dossiers in Switzerland and in the EU is simplified. In Switzerland, authorization takes a few months (apart from the time spent within the company), making it one of the fastest registration procedures in the world. The Swiss approval process also enjoys an excellent reputation internationally, thanks to the strict validation criteria in place and the many first-class hospitals for clinical trials.
Medical devices
Medical devices are not subject to official authorization. For these, Switzerland relies on the requirements of the EU conformity assessment and certification system. To be considered compliant in Switzerland, these medical devices must bear the CE mark of an approved European laboratory, provided all the product information is fully written in three languages (German, French, and Italian). Similarly, a manufacturer located in Switzerland may use the CE mark on its medical devices and sell them on the Swiss market or export them into the EU, EFTA or, Turkey. It should be noted that some of these states require, in addition to the CE marking, registration of certain medical devices and their manufacturers with national authorities. Moreover, non-EU countries sometimes require export certificates from the country of origin, which can be ordered from Swissmedic. In addition, following the announcement of the Swiss government on November 28, 2022, medical devices with a U.S. Food & Drug Administration (FDA) approval are also recognized in Switzerland.
In a nutshell: imports to and exports from Switzerland are facilitated by the agreements that link this country to many foreign countries. However, it is necessary to be familiar with the laws and regulations that apply to the various categories of products in order to ensure that these transactions can be carried out without hindrance.
The Greater Geneva Bern area (GGBa) is the investment promotion agency for Western Switzerland. If you would like to know more about importing foreign products into Switzerland or about other subjects that could help you set up your business, contact us.
Our “Why Switzerland” articles are likely to answer your questions.